Mastering Media pH: The Key to Thriving Greenhouse Crops
Ensuring a stable pH is paramount for greenhouse success, as pH fluctuations can jeopardize crop health and profits. Proactive pH management is the key to ward off nutrient imbalances and promote thriving plant growth.
Understanding pH in Plant Growth
pH Definition
pH, or potential Hydrogen, measures the acidity or basicity of a solution on a logarithmic scale from 0 (most acidic) to 14 (most basic/alkaline), with each increment representing a tenfold difference in hydrogen ion concentration.
pH Balance
pH indicates the balance between hydrogen ions and hydroxide ions in a solution. Lower pH signifies higher hydrogen ion concentration, while higher pH indicates higher hydroxide ion concentration.
Optimal pH Range
Different plant species have varying preferred media solution pH ranges, with the optimal range for maximum growth generally falling between 5.4 to 6.8.
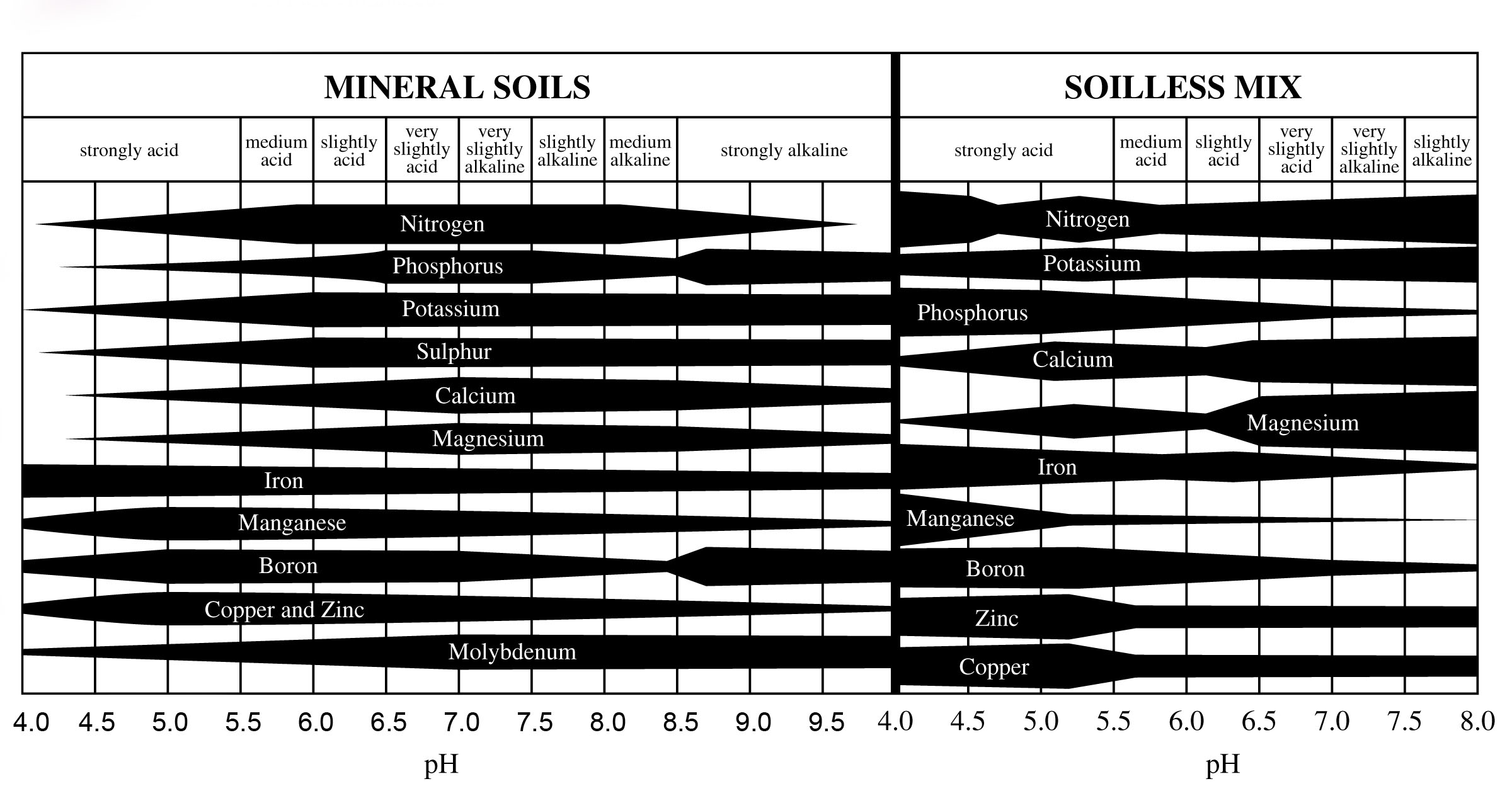
Nutrient Availability
The pH of the media significantly affects nutrient availability for plants. Micronutrients like manganese, boron, zinc, and copper become less available at higher pH levelsand excessively available at lower pH levels. Nutrient availability at different pH levels is dictated by the interaction of ions among the plant roots, media pH, and nutrient solution.
Low pH Effects
When pH is too low, micronutrients become excessively mobile, leading to potential toxicities as plants absorb more than necessary.
High pH Effects
Conversely, high pH limits micronutrient mobility, causing deficiencies as plants cannot absorb enough nutrients.
Recognizing pH Stress
Symptoms like chlorotic leaves and stunted growth indicate nutrient deficiencies due to pH imbalance.
Factors Affecting Substrate pH

Substrate Components
Organic Materials
Peat moss and bark, commonly used in substrates, can be acidic, requiring lime to balance pH. Over time, the acidity of peat and bark may decrease as they break down.
Inorganic Materials
Perlite and vermiculite, though inert regarding pH, can affect substrate porosity, influencing nutrient availability to plants.
Substrate Amendments
Limestone
Adjusts pH before planting, with factors like limestone type (calcitic or dolomitic), particle size, and hardness influencing its effectiveness over time. Proper mixing of different-sized lime particles ensures pH stability throughout the crop cycle.
Fertility Sources
Fertilizer Type
Different fertilizers can impact pH due to their potential acidity or basicity, with nitrate and ammonium forms of nitrogen playing significant roles. See our last post for more information on this (https://www.plantprod.com/news/the-power-of-nitrogen/). Additionally, urea-based fertilizers undergo bacterial break-down, releasing hydrogen ions and affecting substrate acidity over time.
Ionic Balance
The balance of ions in fertilizers affects pH, with nitrate ions contributing to alkalinity and ammonium ions contributing to acidity. This balance is crucial for plant health, as excessive levels of certain ions can lead to nutrient imbalances and pH fluctuations. It’s important to have an adequate level of buffering capacity within your media to mitigate such pH fluctuations. Ensuring an appropriate amount of bicarbonates within your media and irrigation water is a great way to achieve this.
Water Source
Water Quality
Variations in water mineral content can affect pH and plant health, making it an important factor to test annually. For instance, water high in calcium and magnesium carbonates can lead to alkalinity, while excessive iron content can cause acidity.
Water alkalinity
The level of alkalinity in water significantly impacts substrate pH. For instance, comparing two water sources, one with a pH of 9.0 and alkalinity of 50, and the other with a pH of 7.0 and alkalinity of 300, the latter will cause a much greater increase in substrate pH. Generally, water alkalinity plays a more crucial role in affecting substrate pH than the water’s pH itself. You can learn more about this in our water quality blog post https://www.plantprod.com/news/water-quality/

Implementing a pH-testing program and thoroughly assessing preplant production inputs are crucial steps for effective pH management throughout the crop cycle.
Corrective Procedures to Lower Substrate pH
| Use an Acidic Fertilizer | Examples: 20-10-20, etc. Extremely acidic option: 21-7-7 (avoid in cold growing to prevent NH4-N toxicity). Application: Apply through irrigation. Timing: Best when substrate pH starts rising towards the upper limit. |
| Acid Water Drench | Method: Use sulfuric acid to lower irrigation water pH to 4.0 to 4.5. Application: Apply directly to substrate as a drench. Caution: Rinse foliage immediately after application. |
Corrective Procedures to Increase Substrate pH
| Flowable Lime | Application: Apply as a substrate drench with enough volume to leach the pot. Caution: Rinse foliage and avoid injector damage by using rates of 2qt per 100gl of water or less. Split applications can be considered. |
| Hydrated Lime | Caution: Caustic (rinse foliage immediately and avoid skin contact). Application: Apply as a substrate drench with sufficient volume to leach the pot. Note: Rinse foliage immediately after application. |
| Potassium Bicarbonate (KHCO3) | Application: Apply as a substrate drench with enough volume to leach the pot. Caution: Rinse foliage immediately after application. Note: Provides additional K. After application, heavily leach the following day with a complete fertilizer to reduce EC levels and restore nutrient balance. |
