How Do I Adjust My Irrigation Water? – Comparing Acid Choices To Maximize Plant Yield
Whether it’s for ornamental flowers, vegetables or cannabis plants, increasing greenhouse yield comes from more than just your choice of plant fertilizer. Adjusting your irrigation water is just as important and impacts what type of water soluble fertilizer you use. This post will help you determine what acid to add to reduce alkalinity and ensure the right nutrients are getting to your plants including a handy Acid Choice Chart.
In our previous post on how to increase plant yield by adjusting your irrigation water, we discussed the difference between bicarbonates and pH and noted the need for neutralizing high bicarbonate levels in irrigation water. If high levels are not addressed, they can react with calcium and magnesium to form bicarbonate salts, further increasing media pH and removing vital plant nutrients from the solution.
Ideally, you want to see a bicarbonate level between 60-100 ppm (HCO3). When your irrigation water analysis shows a higher level than this, it is time to consider using acid to neutralize enough bicarbonates to bring your count down to this range.
What Acid Should You Add To Your Irrigation Water?
There are several factors to consider when choosing an acid, including safety, nutritional value, and compatibility: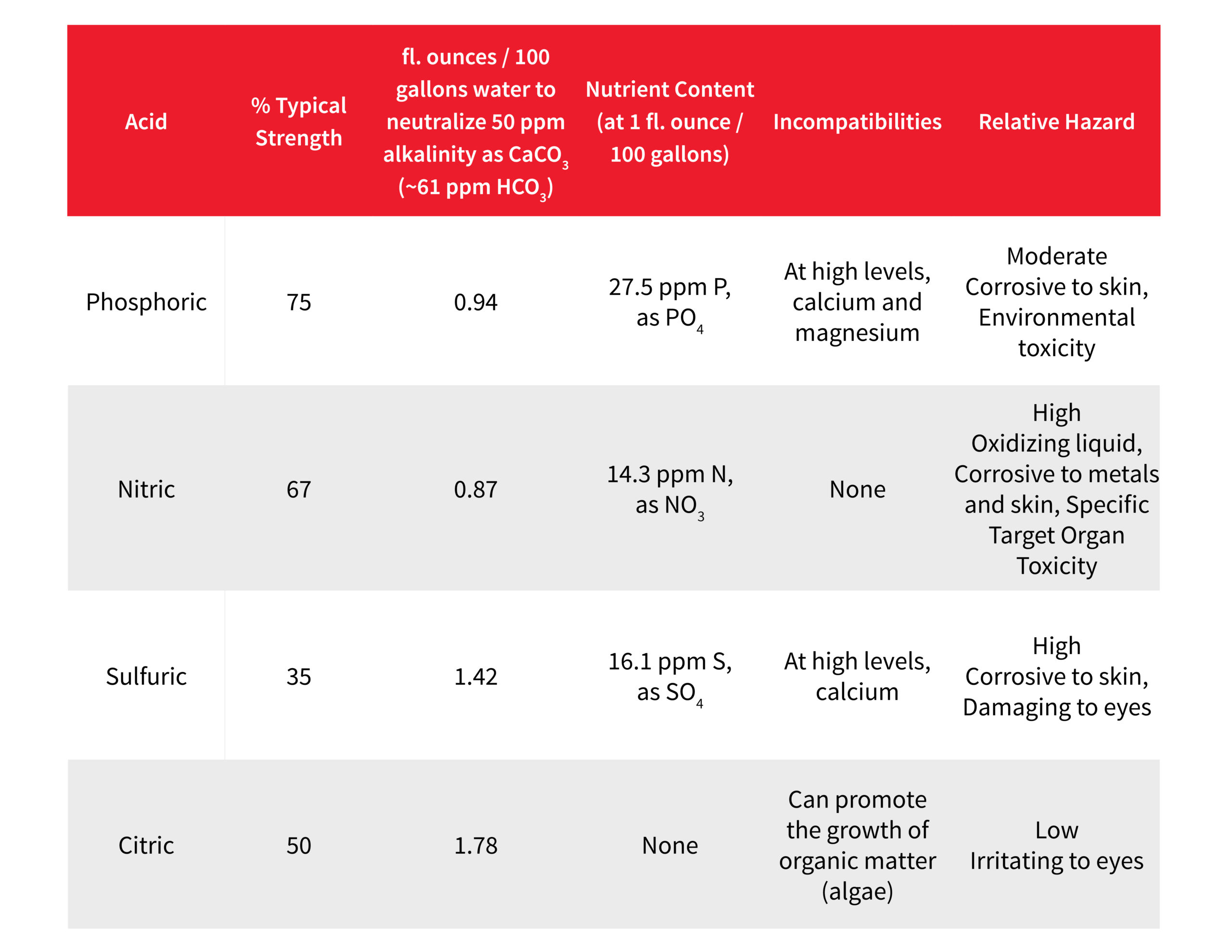
It is important to identify what strength of acid you’re adding to your irrigation water, along with specific gravity to accurately determine the rate you require. Luckily, the University of New Hampshire has created a calculator that does the hard work for you when calculating the right amount of acid to add to your irrigation water – the AlkCalc greenhouse production calculator.
For more information on acid safety, mixing and use, visit the University of Massachusetts’ extension article on Adjusting Alkalinity with Acids.
Plant-Prod is a manufacturer of water soluble fertilizers and water soluble cannabis fertilizers. We formulate with everything from chelated iron, to calcium nitrate and zinc fertilizer in order to help you improve yields while cutting fertilizer costs. Click here to find a Plant-Prod fertilizer supplier near you.
