What’s in Your Water – Alkalinity Water Analysis
Irrigation water quality plays a crucial role in plant health, and one of the key factors to assess is alkalinity. Understanding alkalinity can help growers optimize nutrient management, prevent pH fluctuations, and ensure plants thrive.
Alkalinity vs. pH – What’s the Difference?
Alkalinity may sound like alkaline, but they are not the same. Alkalinity is a measure of water’s capacity to neutralize any acidifying component and resist changes to its pH. Alkaline is a term to describe a water source with a pH greater than 7. While pH measures whether water is acidic or basic, alkalinity determines how stable that pH remains over time. This means that you can have water with high pH (alkaline) and low alkalinity, and vice versa, high alkalinity and low pH (acidic).

Alkalinity vs. Bicarbonates
When reviewing a water analysis report, alkalinity is often presented as calcium carbonate (CaCO3). While this is a helpful indicator of alkalinity, we like to focus on bicarbonate (HCO3) level instead because that is the most prevalent ion in typical fresh water sources at pH 6-10.
- Calcium Carbonate can be easily converted to Bicarbonate:
CaCO3 x 1.22 = HCO3
HCO3 / 1.22 = CaCO3
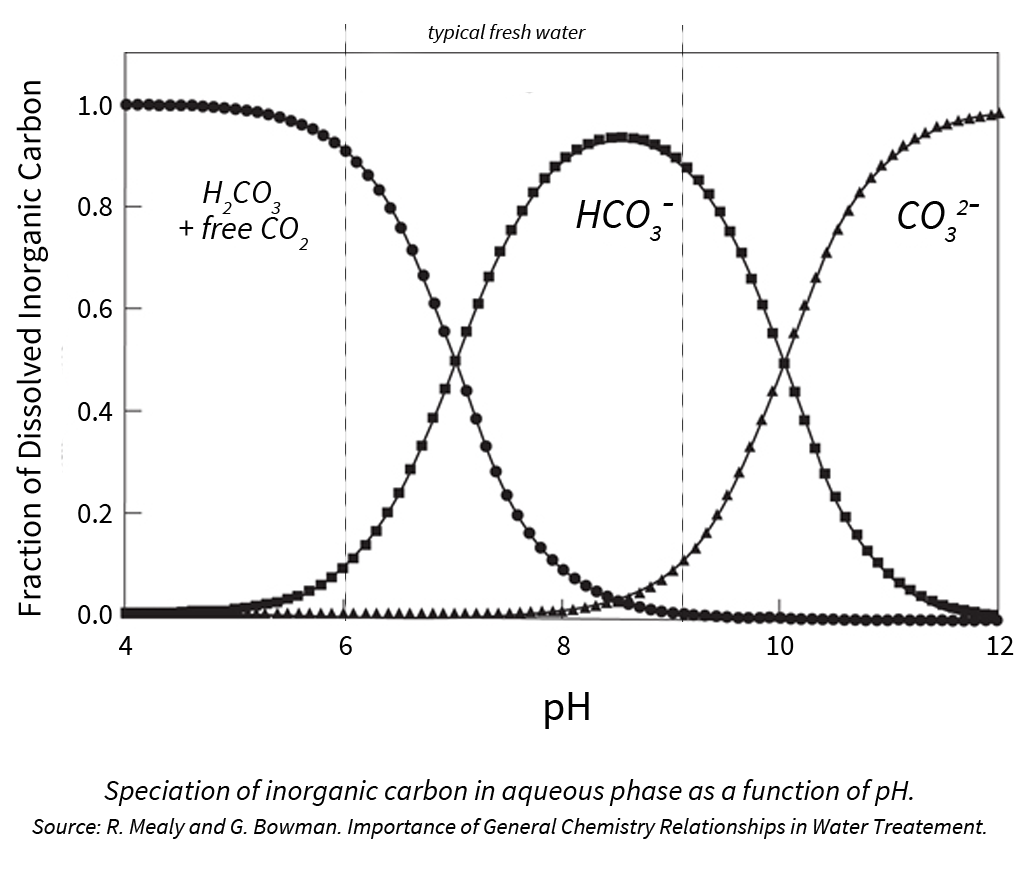
Why Focus on Buffering Capacity?
In the image below, you will notice that the pH of these water sources are similar, and many growers would take the same approach for these 2 very different water sources.
Most growers believe that the pH of the irrigation water impacts the pH of the media. More accurately, it’s the bicarbonate level of the water that allows for changes to media pH.
When looking at these two water sources in more detail, it’s clear that the bicarbonate levels are very different. The RO water has a small amount of buffer to overcome when an acidic source (fertilizer) is added, while the municipal water has a very large amount. To reach the same target pH of 5, you’re going to need a lot more acid for the municipal water source.
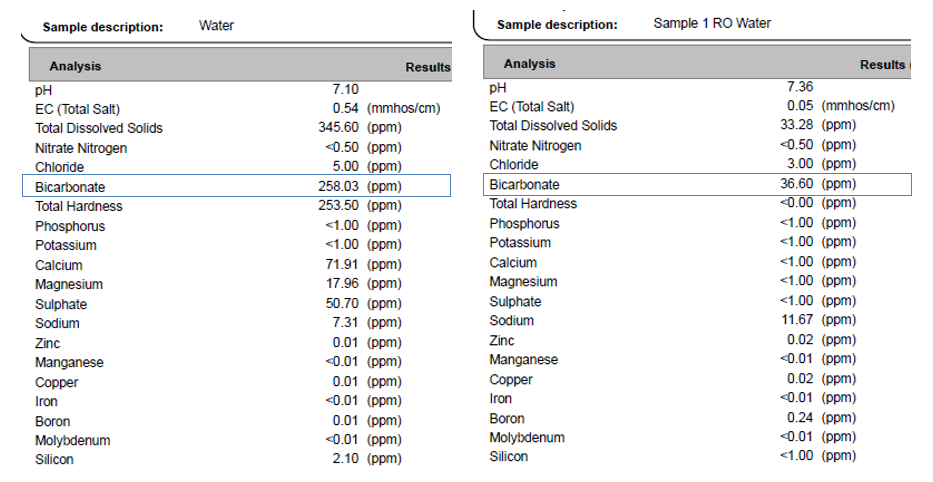
| Initial pH | Initial Bicarbonate (HCO3) | Nitric Acid (67%) Required to Reduce pH to 5.0 |
| 7.10 | 258.03 ppm | 0.270 mL/L |
| 7.36 | 36.60 ppm | 0.038 mL/L |
Adjusting Alkalinity – Acidification vs. Supplementation
The ideal bicarbonate range for most plants is 60-80 ppm, though up to 100 ppm is still manageable. However:
- Below 40 ppm: Additional bicarbonates should be supplemented, typically with potassium bicarbonate.
- Above 120 ppm: Acidification is necessary to reduce bicarbonates to an acceptable level.
When needing to acidify it’s important to also note what other elements you’ll be introducing to your nutrient solution. To determine which acidifier is the right choice for your production, visit our previous blog post on Comparing Acid Choices to Maximize Plant Yield.
Real-World Water Analysis: Municipal vs. RO Water
To illustrate the importance of alkalinity management, let’s compare two water sources: municipal water and reverse osmosis (RO) water.
| Municipal Water | RO Water |
| Bicarbonates: 258 ppm → Needs to be reduced to ~100 ppm using acid. Without adjustment, high bicarbonates can cause excessive pH buffering, affecting nutrient uptake. | Bicarbonates: Near 0 ppm → Needs supplementation with potassium bicarbonate. Without adjustment, water lacks buffering capacity, leading to unstable pH fluctuations. |
Optimizing Water for Plant Health
Whether you need to reduce bicarbonates through acidification or supplement with potassium bicarbonate, proper water analysis is key to a successful growing operation. Regular testing and adjustments help maintain the perfect balance for plant growth.
Stay tuned for the final part of our ‘What’s in Your Water’ series – ‘Undesirables’
